Get Your Free 8 Steps to CRNA Road Map Guide
Just imagine it is 2 AM and you need to learn about a drug, but you don’t have a lot of resources. What can you grab quickly? This episode’s guest has the perfect book for you. Nicole Kupchik, MN, RN, CCNS, CCRN-CMC, PCCN-K has written Critical Care Survival Guide, a concise bedside reference book for anyone working in critical care regardless of experience level. In today’s episode “ICU Cardiac Medications With Nicole Kupchik”, Nicole joins Jenny Finnell to give us access to some of the wisdom from her book, particularly on ICU cardiac medications. They break down the five cardiac medications we commonly see—norepinephrine, epinephrine, Olaparib, milrinone, and dobutamine—and discuss how to titrate them. Tune in to learn more about these cardiac medications, adding more to your CRNA information toolkit!
Get access to planning tools, mock interviews, valuable CRNA Faculty guidance, and mapped-out courses that have been proven to accelerate your CRNA success! Become a member of CRNA School Prep Academy here!
https://www.crnaschoolprepacademy.com/join
Book a mock interview, personal statement critique, resume review and more at https://www.TeachRN.com
Join the CSPA email list here! https://www.cspaedu.com/podcast-email
Send Jenny an email or make a podcast request!
Hello@CRNASchoolPrepAcademy.com
Grab Nicole Critical Care Survival Guide: https://www.nicolekupchikconsulting.com/booksAndCourses/books-bundles/136/critical-care-survival-guide
USE COUPON CODE: FUTURECRNA20 for 20% off!
Watch the episode here
Listen to the podcast here
ICU Cardiac Medications With Nicole Kupchik
I know how painful it is to have burning questions and not have the answers. CRNA School Prep Academy has played a large role in helping to mentor you along your journey to become a CRNA. I also equally value every single one of you because, in turn, you all become mentors in the future. This is why I started Nurses Teach Nurses- Now called TeachRN! I know the power of mentorship and I have seen it change lives. I believe every individual nurse has a role to play in mentoring future generations of nurses.
TeachRN is the only nurse-driven marketplace for nurses by nurses. It was developed by yours truly. I have a passion for mentorship and I know you do as well. If you are looking for mentorship or if you want to become a mentor, you can earn income while doing what you love, which is helping fellow nurses. Head over to TeachRN.com and learn more about how to receive or give the gift of mentorship. Back to the show.
—
We have a very special guest. She’s back to the show, Nicole Kupchik. Welcome, Nicole.
Hey, everyone.
I’m so excited. For those of you who are not aware, Nicole launched her book called Critical Care Survival Guide. This thing is amazing. It is chock full of very organized tabs such as hemodynamics, cardiac medications, different cardiac parameters, respiratory, neuro, renal, endocrine, GI, blood product, IV fluids, and everything else that you can possibly imagine. We’re going to hit five cardiac medications that I feel like you guys can never get enough of. I know it’s a very heavy topic for anesthesia school and in CRNA interviews. I brought Nicole on the show to go over these medications and share some of the wonderful insight that is in this amazing guide that I highly recommend that you pick up. Welcome, Nicole. Thank you for joining us.
Thanks for having me again, Jenny.
It’s always a pleasure and honor to have you. This is exciting because I love getting nerdy and talking about pharmacology and pathophysiology. I know our audience could use a lot of refreshing and deep dive more than just the surface level. I think it’s key, which is what I love about your book. It’s definitely more than just the surface level. You add a lot of value here. One of the first drugs we’re going to talk about is Norepi. Let’s go ahead and get into that.
When I wrote the book, I tried to write it with the idea of there are so many new people in healthcare. One of the things to note is I didn’t call it the Critical Care Nursing Survival Guide. I wrote it so that it could be used by providers, nurses, or whoever is at the bedside. In my 30th year as a nurse, a lot of these drugs are second nature to me. I took a step back and thought, “If I knew nothing about this drug and it’s 2:00 in the morning, what would I need to know?” That’s the way I tried to write the entire book. It’s 2:00 in the morning. I don’t have a lot of resources. Maybe I can trust this woman who wrote this book. What do I need to know? Norepinephrine is the first drug right off the bat. When I became a nurse, it was called, “Levophed, leave them dead” because we used it as a last-ditch effort.
I’ve never heard that. That’s funny.
That was such a 1990s thing. It was one of those drugs that would be like the Hail Mary. If somebody was crashing, we would use dopamine as a first-line drug. We’ve come to find out that in the study it had against dopamine, it came out the winner because it’s got a little bit of beta-1, but more alpha. You’re not going to get the tachycardias like you do with dopamine. Dopamine has got so much beta-1 activation and lots of tachycardias, dysrhythmias, and things like that.
In the book, I try to give a general approach to vasopressors. One of the things I say with vasopressors is when you got somebody who’s hypotensive, you don’t take your time. You crank it and then you dial it back. You need to get their pressure and hopefully, secondary is their perfusion to a point where their kidneys aren’t going into failure, and they’re not having other organ damage and ischemia. I divided Vasopressors into catecholamine-based, and then non-catecholamines. All your catecholamines are quick on, quick off. It’s a 1 to 2-minute onset and about 2.5-minute half-life for all of them. You titrate quickly with those. I try to give some general guidelines around how to use these drugs.
Another thing to remember is with a lot of your vasopressors, especially norepinephrine and possibly vasopressin, you’re probably going to start seeing more peripheral use of them. In sepsis, what we’re finding out is we’ve probably way overdone it with fluid, and now we need to dial it back. You got a vasodilatory-type shock state that it only makes sense to start the vasopressors early.
I put some guidance from the Society of Critical Care Medicine around the peripheral use of vasopressors as well. I try to give some general guidelines but remember, titrate quickly with your vasopressors. Always remember as well that what goes up must come down. We became good at getting vasopressor doses up, but you got to remember these things vasoconstrict. The two organs that vasoconstricts quite heavily are the skin and the GI tract. We don’t have a good way of measuring how those organs are doing. We can at least see the skin, but the GI tract, we cannot.
Norepinephrine
Always think of this when you get to certain doses, especially norepinephrine. Let’s say you use mics per minute. If you’re at 15 to 20 mics per minute, you need to be thinking about second-line vasopressor, either vasopressin or low-dose epinephrine. I try to give guidance around how you use these drugs without being too verbose.
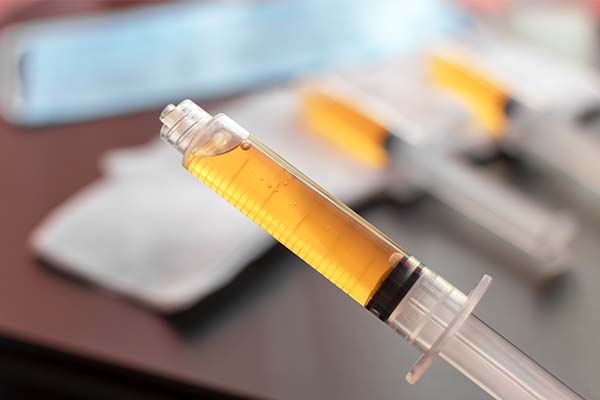
ICU Cardiac Medication: When you get to certain doses, especially like norepinephrine, you need to be thinking about a second-line vasopressor.
That is a very high dose of Epi. I love the fact that you said you want to make sure you’re stabilizing the map and getting the map up, versus taking 1 to 2 minutes to keep titrating very slowly. Time is precious. With the skin aspect of vasopressors, I’ve seen patients turn gray in my ICU years. They get a modeled gray look to them and that’s never good. It’s usually for someone who’s on multiple pressors, but the GI tract is a good point. That toxic megacolon dead gut, when that type of situation arises, there’s usually no coming back from that.
Game’s over. We don’t have a great way to measure that. In the late ’90s, we were using sublingual circulation parameters. Unfortunately, that device got pulled off the market. There were some issues with the manufacturing of it. We have no way of looking at gut circulation. Are we shunting too much blood away from the gut? Always remember in your head, what goes up must come down. Do not be shy about backing off the vasopressors as well.
You mentioned it does beta and alpha. What’s the ratio between beta and alpha? Is it more alpha or more beta?
For Norepinephrine specifically, what I did was I put a little chart in the book where I talk about alpha and beta receptors. Alpha receptors are located in the periphery. Beta one is located in the heart. I always use this little rhyme, “Beta one, you’ve got one heart. Beta two, you’ve got two lungs.” That’s the little rhyme I always use to remember where your receptors are located.
Norepinephrine has a lot of alphas. I gave it 4 pluses on alpha, and then I gave it a 1 to 2 plus on beta one. You’re going to mostly get peripheral vasoconstriction with a little bit of inotrope, meaning you’ll get a little bit of contractility assistance but not so much that the patient gets tachycardic from it. Of course, a lot of it is dose-dependent. Initially, you crank it and then you back off, but you want to keep these drugs at the lowest doses possible because of all the vasoconstriction that they cause. We’ve seen patients lose “ears and nose, fingers and toes” from these drugs because they cause so much vasoconstriction.
I love how you mentioned the heart rate. One thing that’s vital is that the heart rate puts in a lot of oxygen demand. The tachycardia essentially can cause more ischemia because your demand is higher for oxygen and you’re utilizing it much more quickly with tachycardia. Drugs that cause tachycardia can worsen cardiac ischemia. That’s something to keep in mind too. That’s very important as you’re titrating vasopressors that have those effects.
Especially if you’ve got a cardiac patient, what happens during diastole is your coronaries are perfused. If you’ve got a tachycardic patient, that coronary perfusion time is diminished. It’s a great point, Jenny.
Thank you. What would you say you would start someone off on with Levo? Since you mentioned you want to titrate it quickly to get the blood pressure up, how would you titrate it? Based on a standard protocol, isn’t it usually 0.02 to 0.05?
There’s no protocol per se. Hospitals might have a protocol. This is a whole other topic but this is where the joint commission has come in and they’ve tried to regiment and make us protocolize how we titrate vasopressors. When you got somebody who’s hypotensive, you cannot protocolize that. You have to titrate it to effect.
You are afraid to jump all the way. You would go to what you think is necessary. How would you judge where to go?
It depends if you’re using mics per minute or kilos per minute. For example, mics per minute, I might start at 5 mics per minute, and then go up to 10, and then maybe up to 20, but then you can crank it back down. Every minute that your patient is hypotensive or hypoperfused, you are risking organ damage. Specifically, kidneys are big ones. I worry about the GI tract, neuro-perfusion, or cerebral perfusion.
Every minute that your patient is hypotensive or hypoperfused, you are risking organ damage. Share on XThis is the art of being at the bedside. Science tells us to back off as quickly as we can, where we want to use the lowest dose needed. People always say to me, “What’s the best dose?” It’s the one that makes them pee. If you’ve got someone who’s chronically hypertensive, a MAP of 65 probably won’t do it. You’ve got to look at your patients and see what they need.
I love that you said, you got to stick where their baseline is because someone who’s always living with a MAP from the 70s, that’s probably what their body is used to and they need that to perfuse. A MAP of 55 is not going to cut it if they’re living at a MAP of 75 most of the time. Also, the urine output, what is the urine output? I’ve always heard that that’s a later sign. Yes, it’s a sign, but once you see the urine output start to dip, you’re already behind the 8-ball a little bit.
Since we’re talking about urine, I’m going to mention this. At the bedside, we always shoot for urine output of 30 mils per hour. Where did that come from? I have no clue. There’s no science to support 30 mLs per hour. I’m 5’2”. What I always do when I’m live in class is I have the tallest person in the room come stand next to me. I always say, “Who is 30 mLs an hour an appropriate urine output for, me or this person who’s 6’2?” The answer is it’s appropriate for me, probably not the 6’2” person. If you look at any renal guideline, they’re always going to recommend half an mL per kilo per hour. You have to rethink even your urine output goals as well.
I love that you mentioned half an mL per kilo per hour. You guys should remember that. That is the same formula that we go by in the operating room, especially if we’re doing cases like open hearts. If you take the standard 70-kilo patient, then you know it works out. Again, you have to enter 35 mLs per hour or whatever ends up being. That’s where that 30 mL came by. It was the set average weight of 70 kilos. Knowing that that’s not always the average weight per kilo, go by half mL per kilo per hour as what you’re aiming for in urine output.
Obviously, when you’re working with PEDs, that matters too because PEDs are various sizes, so you have to take that into consideration. Also, depending on the patient’s age, your body water is different. Keep that in mind as well. The older you get, you tend to have less body water. The younger you are, a little tiny fluffy little baby has a lot more body water. Understand that dynamic as well.
Is there anything else about Norepi? Let’s talk about the half-life and maybe the mechanism of action. We talked about the alpha and beta. Other than causing vasoconstriction in a positive inotropic effect, is there anything else that we should potentially know about the mechanism of action? Maybe what would be the best line of treatment for certain disease processes like sepsis and things of that nature?
Those are the two main ways that it acts. It’s catecholamine. All your catecholamines are quick on, quick off. It’s about a 1 to 2-minute onset with about a 2-minute half-life. It is the first-line vasopressor in most guidelines, specifically for sepsis as well. One of the ideas is that you want to think about a second-line vasopressor early, especially in the setting of sepsis. Our guide was always when you get to about 15 to 20 mics per minute and then move to 0.2 spots if you’re doing mics per kilo per minute. Think about adding that second-line vasopressor. Vasopressin is an anti-diuretic hormone, so it’s going to act through your vasopressin 1 and vasopressin 2 receptors. It’s going to cause vasoconstriction, but it’ll also cause the body to hold on to sodium and water.
The half-life of vasopressin is about 10 to 20 minutes. It’s not one of those drugs that you actively titrate. You just put it on. Especially in sepsis, there’s some thought that people are ADH deplete. There are some benefits there. There are some thoughts that there is a synergy between norepinephrine and vasopressin. It works in a different mechanism. The sepsis guidelines with the update in 2021 recommended starting at 0.03 units per minute versus 0.04. The reason was that there were concerns with gut ischemia. For any vasopressor, you’ve got to worry about gut ischemia, which is difficult to identify and measure in patients. Norepinephrine, in most guidelines, is going to be your first-line vasopressor, but remember, it is a potent drug. It is super potent.
In sepsis, unfortunately, because of the pandemic, cost, timing, and everything else, another great drug is angiotensin II. Giapreza is the other name for that or angiotensin II. Some facilities use it as a last-ditch effort. Here’s what I’m going to say about that. If any drug you use as a last-ditch effort is not going to work, it becomes a self-fulfilling prophecy. You’ve got somebody who’s just about dead and you’re like, “Let’s start this drug. That drug doesn’t work.” Yeah, because they were about dead when you started it. It’s not going to work in that situation.
Any drug you use as a last-ditch effort is not going to work. It becomes a self-fulfilling prophecy. Share on XMy experience with angiotensin II started early. It got FDA approval as a first-line vasopressor, but a lot of people use it as the second-line, cost being a concern, but it’s a great drug. Angiotensin II is a very potent vasoconstrictor. It’s something that naturally occurs in our body. In fact, in heart failure, we block angiotensin II because it’s such a potent vasoconstrictor. That’s another drug for sepsis, which as a CRNA, you’re probably not dealing directly with sepsis, but you have septic patients in the OR. That’s another drug that’s great to use as well. It’s dosed in nanograms, which is a different way of dosing than what we’re used to.
Epinephrine
Let’s get into epinephrine. That’s a big gun as well, but something that is critical to understand. Talk about the mechanism of action and the first receptor sites of epi, and maybe even how it’s dose-dependent.
Epinephrine, the way it works all depends on what dose it’s at. When you’ve got lower doses, it’s mostly going to act as a positive inotrope. There’s a lot of cardiac dysfunction in sepsis, and low-dose epinephrine is going give you more positive inotrope, so more of that beta-1 effect. You’re going to get increased strength of contractility. As you get on higher doses, it’s going to vasoconstrict the periphery.
This is another wild thing I’ve seen with epinephrine. I saw this a few years back. Sometimes with epinephrine what you might see, especially in a septic patient, is this massive increase in lactate. One of the thoughts is that it causes type-B lactic acidosis, and then it frees up lactic acid. I’ve heard some providers say, “The whole idea is that the body may use it as metabolic fuel.” How well-supported that is, I’m not totally sure. You might see this big upswing in lactate levels because it causes type-B lactic acidosis, not type-A.
The type A lactic acidosis, we see high lactates because of hypotension or hypoperfusion, whereas type B lactic acidosis is not life-threatening. You just get an elevation in lactic acid. We see it with albuterol, epinephrine, thiamine deficiencies, metformin, and different drugs like that. Just be aware if you are using epinephrine, you might see this escalation of lactate levels. The other thing to watch out for is hyperglycemia. With epinephrine, you see some insulin resistance and you may see hyperglycemia. Even non-diabetic patients may need some insulin when they’re using epinephrine infusions.

ICU Cardiac Medication: Be aware if you are using epinephrine, you might see an escalation of lactate levels.
That’s a good point to point out about insulin and hyperglycemia. I’m curious about the lactate, you say that the levels elevate. Are they differentiating between A and B in the test? I don’t remember them ever doing that. I wonder if they would see that as alarming when you said type B is not alarming, but how could you differentiate? Just because they went on a drug, and now it’s increased?
I’ll give you an example of a patient who I took care of not too long ago. It was a patient who was septic. They were on norepinephrine and vasopressin, and then we added epinephrine. Before we added the epinephrine, the last lactate level was right around three, so it was still elevated. This is a patient who was in septic shock. When we added the epinephrine, the lactate levels went up to 9 and peaked at 12, but the patient looked better. They were peeing. They were less modeled. They overall looked better and their SvO2 was better, so we did a spot check. The patient had a triple-lumen catheter. What we did was we did a spot check. Before we started the epinephrine, I want to say the SvO2 was 45%-ish, somewhere around there. Once we added the epinephrine, the SvO2 went up to almost 60%.
The patient physiologically looked better, but they had these crazy high lactate levels. They were peeing. They were doing much better. Cardiac output was up. SvO2 was better. We’re looking at these like this patient does not look like a lactate of twelve. This is actually type-B lactic acidosis that caused the body to free up this lactate. Anyway, we let it be, and then the provider wasn’t comfortable with it, so we turned off the epinephrine and used dobutamine instead, and the patient’s lactate levels cleared.
That’s a great explanation and scenario that you guys should listen to on repeat because especially, if all you went by was that lab value, you would think that he was doing way worse. Clearly, you shared that they painted the picture, but making note of where they start is key before you start the new drugs. Knowing where the lab values, what the last ABG looked like, and what the SvO2 was before you start a new drug is key to being able to say, “What’s the cause and effect relationship?” I think that’s a great point to make, so thank you for saying that.
Epi, I know you mentioned the different dosing, cardiac dose, point 0.01 to 0.02, we usually did 0.03. Obviously, the more you titrate up, the more you’re going to get the squeeze. With the alpha receptors, increased lactate levels, and pulmonary edema, what is that cause, just from the increased vasoconstriction and the pulmonary system being affected by that?
I think a lot of it depends on what’s happening with the patient, but it could be the demand is higher. There’s more resistance toward the lungs. PVRs go up with this drug, depending on the dose. A lot of it depends on the patient’s scenario and what dose you’re at. It’s one of those things to watch out for. At low doses, I’m not as worried about it, it’s higher doses.
You mentioned myocardial ischemia, which we already briefly covered with tachycardia.
It’s all demand.
Phenylephrine
Let’s move on to anesthesia’s favorite drug, phenylephrine.
I was joking about it, Jenny. When I teach, I always try to be funny and silly. I always say, “Who loves phenylephrine? Anesthesia.” In fact, they carry mystery unlabeled syringes of it in their pocket. They come into your post-op patient’s room, pow-pow, and leave. You’re like, “Why is the pressure 220/120?”
This is bad too. I was talking to a PACU nurse on a podcast episode. I have never done this, but when I was in clinical training, I saw CRNAs do this. I’m like, “What? This is not right.” Guys, don’t do this. They would go to the PACU and they would put 500 mics of Neo in whatever is left in their saline bags. If their bag had 500 mLs left, they’d squirt the Neo into that. They would give a report to the PACU nurse and they would have a little tiny mini infusion of Neo going. It’s not okay. You’ll see a lot of things in practice. If the light bulb goes off, you have to know that that’s not a good practice. PACU nurses are very well aware that sometimes anesthesia does stuff like that. Levo is a great drug, but let’s talk about it.
That’s interesting.
I feel weird even saying that like, “This is so not okay,” but I just know it’s done. Let’s talk about the mechanism of action and the effects on different receptor sites and things, and what makes it different from Levo or Epi.
Phenylephrine is pure alpha. It’s going to give you peripheral vasoconstriction. It’s not going to give you inotropes, you’re not going to get extra squeeze. It’s going to increase the workload of the heart because it’s increasing afterload in the SVR. In a lot of patients, that’s okay because they’re vasodilated and that’s why you’re giving it, especially post-anesthesia. It’s going to work a lot differently now. In some patients, you might see reflexive bradycardia from phenylephrine because there’s so much peripheral vasoconstriction that they have basically a parasympathetic response to it.
One of the things I’m going to say though is that it’s not recommended in septic patients. There are some data that perhaps it’s unsafe in septic patients. It would be interesting to dig into the why’s. I honestly have never dug that deep to figure out why in sepsis. Sepsis was always more epinephrine, vasopressin, low-dose epi, and dobutamine. Remember that it’s pure alpha, so it’s going to tighten up the periphery.
My understanding is we don’t ever use it in the NICU. We were always using Levo. My girlfriend who works in the surgical ICU was using Neo drips all the time. I’m like, “That’s weird.” I remember the thinking is because in sepsis and a lot of those types of patients, you have so much cardiac dysfunction. When you’re squeezing the pipe, the failing pump can’t overcome the afterload and increase SVR, so you can worsen the heart failure and cause right-side of heart failure and back up the blood. You needed that positive inotrope with the Levo to overcome the increased SVR. That’s why Levo was always a better choice in sepsis versus someone who is more dealing with a vasoplegic injury and their pumps are okay.
It’s one of those drugs that’s so important that you have to have volume. I stress this all the time when I train and I precept, because I see that so quickly they go for that without assessing first. It’s because they’re losing blood or volume or insensible loss, which in a big GI case, you lose a lot every hour. Your third space too. There are a lot of things going on. You cannot give a drug like Neo without adequately replacing the volume that the patient has lost or is losing or needs. I think about it like a closed pipe that’s collapsed, so you’re trying to squeeze it more and there’s nothing inside the pipe to squeeze.
That would be super true for any of the vasopressors. You can’t use this in a patient who’s got a volume issue. You’ve got to replace the volume. Our patients get so sick and they third space a lot of fluid. It can be challenging at the bedside to decide, “Is this patient dry or not? They’re fluid-overloaded, but where is the fluid?” That’s where the big challenge comes in. That’s where using things like the passive leg raise test with stroke volume devices or even giving the patient measuring stroke volume.
Giving a fluid bolus in a very short period of time, over a minute to three minutes to see what their stroke volume does. Are they going to be fluid or preload-responsive? If not, then you use a pressor. From a hemodynamic standpoint, that’s a whole other podcast episode, but from a hemodynamic standpoint, we have got to start paying attention to this because fluid overload is not benign. It causes a lot of issues down the road. To that point, you also can’t use a pressor if you don’t have enough volume.
You have to figure out what it is that they need, and then go from there. Obviously, sometimes you do your best and you don’t always have evasive lines to do these things. For example, in anesthesia, if you don’t have any way to measure what their stroke volume is, and if it’s not in the ICU with all these fancy pretty things, you give a little fluid bolus to see if they’re responsive. If they are, then that’s what they needed. If they’re not, then they’re vasoplegic.
It’s about figuring out what they need, but being careful that you’re not whopping in with a liter. I’m saying you test them with a 250 bolus or maybe 500 tops. Even 250, you can tell if you give someone 250, if they’re going to be fluid-responsive. Also understanding the pathophysiology process. What are their comorbidities? Also, what’s the process that’s going on? What’s the disease process, or in surgery, what’s the surgery? What are their losses? Take all that into account before you assume that they need one thing and try to be the scientist and figure it out.
One thing I’ll pipe in and say is heart rate, blood pressure, and central venous pressure do not tell you if your patient’s going to be fluid-responsive. The science is super clear on that. If you’re using entitled CO2, if a patient has an art line, you can look at pulse pressure differences. I would say to all of the CRNAs or future CRNAs, this is something I wish anesthesia would massively advocate for. Even if it’s non-invasive volume measures when you’ve got these big abdominal surgeries, or whatever the case might be, should we be using hemodynamics and not just guessing? If you’re using heart rate and blood pressure to decide if your patient is fluid-responsive, you are totally guessing.
If you're using heart rate and blood pressure to decide if your patient is fluid-responsive, you are totally guessing. Share on XUsually, in the bigger cases, we do have different ways to measure, but I think a lot of room for improvement can be done in those cases. I would love and encourage those of you tuning in, if you’re going to be thinking about a DNP project, this could be a light bulb going off saying, “Let’s focus here and see if there’s a way that we can improve patient outcomes by doing some stroke volume measurements during big abdominal cases or other cases where there are lots of fluid shifts going on in third spacing.” Even the patients who come down to the OR, who already have a lot of diseases going on, and who are at high risk. A septic gallbladder could be one of them. Those can go bad so quickly that having those types of measurements in the OR in real time would be a lifesaving measure.
Let’s see what else is here. We talked about reflex bradycardia. That definitely does happen. It depends on the dose that you give and how significant the bradycardia can be. They never seem to go asystolic. I’ve given pretty high doses myself and I’ve never seen them go asystolic, but it does significantly slow them down. Metabolic acidosis, is this related to the increase in SVR? What would that be caused by?
It could be multifactorial. Definitely, it could be a worsening shock state. We see metabolic acidosis in shock so frequently. A lot of that is multifactorial, so understanding if you’re using these vasopressors, you often do see metabolic acidosis.
Milrinone
Could it be from a VQ mismatch of some kind? Let’s move on to Milrinone. Maybe I’m speaking for myself, but I feel like I wasn’t super familiar with it. I knew about it in the ICU, but I didn’t use it a lot. You don’t even use it a lot in anesthesia, but when you do, it’s a very important drug and it can be lifesaving. Let’s talk about its uses and how it works.
Milrinone is a Phosphodiesterase Inhibitor. It has a little nickname called an inodilator. It’s a positive inotrope that’s going to increase contractility by inhibiting cyclic AMP at the cellular level. It also vasodilates. In a lot of situations, that’s good because you’re getting a positive inotropic, and then less resistance to pump against. Here’s the deal. I was on a webinar a few weeks ago and this cardiac surgeon was talking about milrinone versus dobutamine. He’s like, “I love milrinone, but I’ve always got to bring her best friend with her, norepinephrine, because the vasodilation can cause hypotension.”
Dobutamine is a catecholamine inotrope and it’s quick on, quick off because it’s a catecholamine. Whereas milrinone has a lot longer half-life. The half-life is about two and a half hours. When you use it, it’s an awesome drug. It works great, but you’ve got to have some pressure to play with or the willingness to start a vasopressor if needed. It’s an awesome drug.
About 2.5 to 3 years ago, it was studied head-to-head against dobutamine in shock states and it came out with pretty equivocal results compared to dobutamine. Although in a lot of shock states, we’re more comfortable with dobutamine because of the catecholamine nature and quick on, quick off. If they get hypotensive, you can just back off and the half-life is a few minutes. Milrinone, I love using in exacerbated heart failure patients. It works beautifully because you get the dilation. It’s a lot more significant than what you get with dobutamine. Dobutamine, you do get some vasodilatory effects but it is far more pronounced with Milrinone.
I agree. They are usually best friends. We would use it after our open heart and we typically always had to have Levo on with it. We had to be careful when we titrated it because the half-life is so lengthy that you go so slow and start so little with that drug. If you have to get it off, then now you’re stuck with the effects for some time, but it’s a great drug when it works. We would use it a lot in pressure-dependent patients who have pulmonary hypertension. They were great patients for it.
For anyone who’s got right heart issues, I love it in right heart issues because it keeps the blood forward flowing to the left. It does definitely work great with right ventricular dysfunction.
It is excreted or metabolized by the renal system. If you have renal failure, is it a complete contraindication or you just be careful with it?
We use less of a dose a lot of times. That’s what I’ve seen clinically, and then if there are deep concerns, they can use dobutamine instead. I haven’t even seen the combination. Instead of using milrinone, they might use dobutamine with a dose of nitroprusside to get the afterload effect. You can do a couple of different things.
We mentioned here in your book hypokalemia and thrombocytopenia.
Hypokalemia, a lot of times, you see increased contractility. Potassium can come down. With dobutamine and milrinone, it’s always good to know what the potassium is, and then anticipate that it can go down. The other thing is often you’re using these drugs with diuretics as well. You’re using them with loop diuretics because they’re in heart failure. It’s another reason to keep a close eye on potassium.
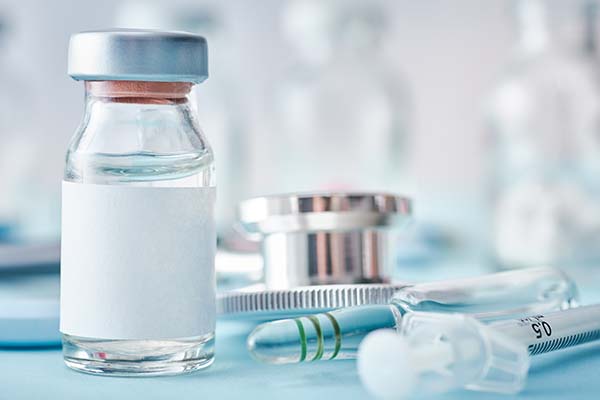
ICU Cardiac Medication: With dobutamine and milrinone, it’s always good to know what the potassium is and then anticipate that it can go down.
I was not familiar with the thrombocytopenia side effect of it. Is that for long-term use maybe?
I’d have to honestly look up the mechanism of why. I don’t remember off the top of my head why, but it is listed in almost every textbook you look at as a side effect. I’d have to dig and look up why.
Dobutamine
That’s fine. My guess is it is probably over a period of time. I feel like in most drugs, the longer you’re on it and dose-dependent and how high of a dose is usually how a lot of these potential side effects arise. The last one we’re going to talk about on this show is dobutamine. We’ve already mentioned it quite a bit because it sounds like it’s a great adjunct for various types of needs, whether that would be sepsis or whether you need a little bit of extra squeeze with some dilatation.
We use it in cardiac dysfunction and heart failure or if you’ve got right or left heart failure. We use it in lots of different situations. A couple of things about dobutamine are it’s quick on, quick off. This is not one you titrate fast. Even though it’s a catecholamine, it’s a catecholamine inotrope. For any inotropic drug you use, you want to start the drug and give the patient some time.
When you titrate this drug, you’re titrating two measures of cardiac output. Not blood pressure. I’m going to be super clear about that. You do not titrate this drug to blood pressure because I’ve seen that done clinically erroneously. You want to see if there’s urine output picking up. Is their skin looking better? If you have a central line, a lot of times what we’ll do is spot-check ScvO2, Saturation of Central Venous Oxygen where you draw blood from the triple lumen, get your baseline, and see where you’re at. When you start dobutamine, you should see the ScvO2 go up.
Identify its effectiveness by measures of contractility. If you’re lucky enough to have a cardiac output and stroke volume measure, those numbers should go up. The SVR probably is going to come down because there is some vasodilatory effect to it. Most patients will start 2 mics per kilo per minute, then you might go up to 3, and see how they do. If they need more, then you might go up to 5. You want to give this drug some time to work. It’s not a fast-titrating drug. Your vasopressors for hypotension, you go fast but you’ve got to come back down fast too. Your inotropes, you’re going to give the patient more time. How much time? I don’t know. We might start it and give them 15 to 30 minutes, see how they do, and then reassess from there. Again, reassessing a contractility measure is not by blood pressure.
Thank you for clarifying that.
Watch the tachycardia. They can get tachycardic from this drug. That’s one thing you’ve got to watch out for. Watch the tachycardia with this drug.
I see here dyspnea. That’s probably also from tachycardia, like shortness of breath. “I have an SVT. I can’t breathe.” Thank you so much, Nicole. This was enlightening. I had fun discussing all these different drugs with you. I hope our audience enjoyed it as well. Let people know how to find this book. Go to the website, which is NicoleKupchikConsulting.com.
I have a tab for it on my website. I’m excited. It was crazy. I did not realize what a need there was in healthcare for this book. We’ve sold in the first couple of days over 1,000 books. We’ve been head down trying to get these books fulfilled. For anyone who works with critically ill patients, I’m hoping this becomes your go-to. In the future, I plan on making an app. I need some time to figure all that out, but I’m hoping to have an app for it and keep making enhancements and updating. A couple of new vasopressors are being studied right now. We’ll see what the future shows with those, but I’ll update it as needed.
Thank you so much. Follow Nicole on Instagram. She always posts wonderful things and shares a lot of insight there. Make sure you’re following her. As always, thank you. I appreciate you for tuning in and we’ll see you guys in the next episode. Thanks, Nicole.
Important Links
- NursesTeachNurses.com
- Nicole Kupchik
- Critical Care Survival Guide
- Society of Critical Care Medicine
- Instagram – @NicoleKupchik
Get access to planning tools, mock interviews, valuable CRNA Faculty guidance, and mapped-out courses that have been proven to accelerate your CRNA success! Become a member of CRNA School Prep Academy here!
https://www.crnaschoolprepacademy.com/join
Book a mock interview, personal statement critique, resume review and more at https://www.TeachRN.com
Join the CSPA email list here! https://www.cspaedu.com/podcast-email
Send Jenny an email or make a podcast request!